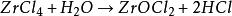Zirconium (IV) chloride, i ʻike ʻia ʻozirconium tetrachloride, loaʻa ke kumu molekala ZrCl4 a me ke kaumaha molekala o 233.04. Hoʻohana nui ʻia ma ke ʻano he analytical reagents, organic synthesis catalysts, waterproofing agents, tanning agents.
inoa huahana:Zirconium chloride;Zirconium tetrachloride; Zirconium(IV) chloride
Kaumaha Molekala:233.04
EINECS :233-058-2
Puni paila:331 (sublimation)
Māmā:2.8
ʻO ke kumu hoʻohālikelike:ZrCl4
CAS :10026-11-6
Lae hehee:437
Hiki ke hoʻoheheʻe ʻia i ka wai: Soluble i ka wai anuanu
1. Waiwai
ʻAno kino a me kemika
1. ʻAno: Keʻokeʻo aniani keʻokeʻo a i ʻole ka pauka, maʻalahi deliquescent.
2. Ka helu hehee (℃): 437 (2533.3kPa)
3. Kohu paila (℃): 331 (sublimation)
4. Māmā pili (wai=1): 2.80
5. Pumi mahu (kPa): 0.13 (190 ℃)
6. Pumi koʻikoʻi (MPa): 5.77
7. Solubility: Hiki ke hoʻoheheʻe ʻia i ka wai anuanu, ethanol, a me ka etera, hiki ʻole ke hoʻoheheʻe ʻia i loko o benzene, carbon tetrachloride, a me carbon disulfide.
Maʻalahi e hoʻomoʻa i ka wai a me ka wai, hydrolyzed i loko o ka hydrogen chloride a me ka zirconium oxychloride i ka ea haʻahaʻa a i ʻole ka hopena wai, ʻo ka hoohalike penei:
Paʻa
1. Paʻa: Paʻa
2. Nā mea i pāpā ʻia: ka wai, nā amine, nā ʻona, nā waikawa, nā esters, nā ketones
3. Nā kūlana e pale aku ai i ka pilina: ea humid
4. Polymerization pilikia: non polymerization
5. Decomposition huahana: chloride
2.Aplication
(1) Hoʻohana ʻia no ka hana ʻana i ka zirconium metala, nā puaʻa, nā mea hoʻoheheʻe wai lole, nā mea hoʻonaninani ʻili, etc.
(2) Hoʻohana ʻia no ka hana ʻana i nā pūhui zirconium a me nā mea hoʻohuihui metala organik, hiki ke hoʻohana ʻia e like me ka solvent a me ka mea hoʻomaʻemaʻe no ka metala magnesium remelted, me nā hopena o ka wehe ʻana i ka hao a me ke silika.
3.Synthetic ala
E kaupaona i ka zirconia a me ka kalapona kalapona ʻeleʻele e like me ka ratio molar o ke ana ʻana, hui like a hoʻokomo iā lākou i loko o ka moku porcelain. E hoʻokomo i ka waʻa porcelain i loko o kahi paipu porcelain a hoʻomehana iā ia i 500 ℃ i loko o kahi kahawai kinoea chlorine no ka calcination. E hōʻiliʻili i ka huahana me ka hoʻohana ʻana i kahi pahele ma ka lumi wela. Ke noʻonoʻo nei i ka sublimation o ka zirconium tetrachloride ma 331 ℃, hiki ke hoʻohana ʻia kahi paipu 600mm lōʻihi e hoʻihoʻi hou iā ia i loko o kahi kahawai hydrogen ma 300-350 ℃ e wehe i nā oxides a me ka ferric chloride i ka zirconium chloride.
4. Ka hopena i ke kaiapuni
Nā pōʻino olakino
Ke ala hoʻouka: inhalation, ingestion, pili ʻili.
ʻO ka pōʻino olakino: Hiki i ka inhalation ke hoʻoulu i ka hanu, mai ale. He ukiuki ikaika kona a hiki ke hana i ke ahi a me ka poino o ka maka. Hiki i ka hoʻohana waha ke hoʻoulu i ka ʻeha ma ka waha a me ka ʻāʻī, nausea, luaʻi, nā wai wai, nā ʻōpū koko, hāʻule, a me nā ʻeha.
Nā hopena maʻamau: Ke kumu o ka granuloma ʻili. ʻO ka ʻeha ʻeha i ka ʻōpū hanu.
Toxicology and Environment
ʻO ka ʻawaʻawa ʻawaʻawa: LD501688mg/kg (hoʻohana waha i nā ʻiole); 665mg/kg (iole waha)
Nā hiʻohiʻona pōʻino: Ke kau ʻia i ka wela a i ʻole ka wai, decompose a hoʻokuʻu i ka wela, hoʻokuʻu i ka uahi ʻawaʻawa a ʻino.
Huahana (decomposition): hydrogen chloride.
ʻO ke ʻano o ka nānā ʻana i ka hale hana: Plasma spectroscopy (NIOSH method 7300)
Ka hoʻoholo ʻana i ka ea: e hōʻiliʻili i ka hāpana me kahi kānana, e hoʻoheheʻe me ka waika, a laila e ana me ka spectroscopy absorption atomic.
Nā kūlana kaiapuni: ʻOihana Palekana a me ke Ola Ola (1974), ʻAwelika Kaumaha 5.
Leakage pane pilikia
E hoʻokaʻawale i ka wahi i hoʻohaumia ʻia, e hoʻonohonoho i nā hōʻailona hōʻailona a puni, a e ʻōlelo aku i nā limahana lapaʻau e hoʻokomo i ka mask gas a me nā lole pale kemika. Mai hoʻopili pololei me ka mea i hoʻokuʻu ʻia, e pale i ka lepo, kahili pono iā ia, e hoʻomākaukau i kahi hopena o ka wai ma kahi o 5% a i ʻole ka waikawa, e hoʻohui mālie i ka wai ammonia dilute a hiki i ka ua, a laila e hoʻolei. Hiki iā ʻoe ke holoi me ka nui o ka wai, a hoʻoheheʻe i ka wai holoi i loko o ka ʻōnaehana wai. Inā nui ka leakage, e wehe iā ia ma lalo o ke alakaʻi ʻana o nā limahana loea. ʻO ke ʻano o ka hoʻolei ʻōpala: E hoʻohui i ka ʻōpala me ka sodium bicarbonate, kāpī ʻia me ka wai amonia, a hoʻohui i ka hau paʻi. Ma hope o ka pau ʻana o ka hopena, holoi me ka wai i loko o ka ʻōpala.
Nā hana pale
Pale hanu: Ke ʻike ʻia i ka lepo, pono e hoʻohana i kahi mask gas. E hoʻohana i kahi mea hoʻomaha hanu i ka wā e pono ai.
Palekana maka: E hoʻohana i nā maka aniani palekana.
Nā lole pale: E ʻaʻahu i nā lole hana (i hana ʻia me nā mea anti-corrosion).
Pale lima: E hoʻohana i nā mīkina lima.
'ē aʻe: Ma hope o ka hana, e ʻauʻau a hoʻololi i nā lole. E mālama kaʻawale i nā lole i haumia i nā mea ʻawaʻawa a hoʻohana hou ma hope o ka holoi ʻana. E mālama i nā ʻano maʻemaʻe maikaʻi.
Nā hana kōkua mua
Pili i ka ʻili: Holoi koke me ka wai no 15 mau minuke. Inā he kuni, e ʻimi i ka lāʻau lapaʻau.
ʻIke maka: E hāpai koke i nā lihilihi a holoi me ka wai kahe a i ʻole ka paʻakai physiological no 15 mau minuke.
Inhalation: E hoʻoneʻe koke mai ke kahua i kahi ea hou. E mālama i ka ʻōpū hanu ʻole. E hana i ka hanu ʻana inā pono. E ʻimi i ka lāʻau lapaʻau.
Ingestion: Ke ala ka mea maʻi, holoi koke i ko lākou waha, mai hoʻoulu i ka luaʻi, a inu i ka waiū a i ʻole nā hua keʻokeʻo. E ʻimi i ka lāʻau lapaʻau.
ʻO ke ʻano hoʻopau ahi: ʻūhā, carbon dioxide, one, pauka maloʻo.
5.Storage ala
E kūʻai i loko o kahi hale kūʻai maloʻo, maloʻo a maikaʻi. E mālama i nā huna ahi a me nā kumu wela. Pono e hoʻopaʻa ʻia ka pahu a pale ʻia mai ka makū. Pono e mālama kaʻawale ʻia mai nā waikawa, nā amine, nā ʻona, nā esters, a me nā mea ʻē aʻe, a pale i ka waiho ʻana. Pono e hoʻolako ʻia ka wahi mālama me nā mea kūpono e loaʻa ai nā leaks.
6 hoʻoponopono kemika helu helu
1. Waiwai kuhikuhi no ka helu ʻana i ka hoʻohālikelike hydrophobic (XlogP): ʻAʻohe
2. Ka helu o nā mea hāʻawi paʻa hydrogen: 0
3. Ka helu o nā mea hoʻopaʻa paʻa hydrogen: 0
4. Ka helu o ka paʻa kemika hiki ke hoʻololi: 0
5. Ka helu o nā tautomers: ʻAʻohe
6. Topological mole polarity ili ili: 0
7. Ka helu o nā ʻātoma kaumaha: 5
8. Ka uku o ka ili: 0
9. Paʻakikī: 19.1
10. Ka helu o nā ʻātoma isotope: 0
11. E hoʻoholo i ka heluna o nā kikowaena hoʻolālā atomika: 0
12. Ka helu o na kikowaena hana atomika maopopo ole: 0
13. E hoʻoholo i ka helu o nā kikowaena stereo hoʻopaʻa kemika: 0
14. Ka helu o nā stereocenter hoʻopaʻa kemika maopopo ʻole: 0
15. Ka helu o nā pūʻulu pili covalent: 1
Ka manawa hoʻouna: Mei-25-2023