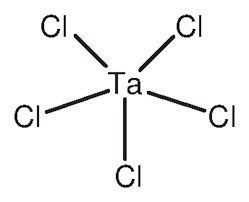
ʻO Tantalum chloride, i kapa pinepine ʻiatantalum Chloride(TaCl₅), he keʻokeʻo, crystalline inorganic pūhui e pāʻani ana i kahi hana koʻikoʻi i nā ʻoihana kemika a me nā ʻoihana uila. Ma kona ʻano maʻemaʻe (formula TaCl₅) he pauka keʻokeʻo a lawelawe ʻo ia ma ke ʻano he mea hoʻomaka no kahi ākea o nā kemika hoʻokumu i ka tantalum. ʻO ka TaCl₅ ka mea hoʻoikaika nui - hiki ke hoʻoheheʻe ʻia i ka ea e hana i ka tantalum oxychloride a ma hope o ka tantalum pentoxide - no laila pono e mālama mau ʻia ma lalo oanhydrous(wai-wai) kūlana. ʻO kēia ʻano naʻau i ka makū, ʻo ia ka TaCl₅ e mālama mau ʻia a hoʻouna ʻia i loko o nā pahu i hoʻopaʻa ʻia a maloʻo.
Ma kēia ʻatikala, e ʻimi mākouʻelua mau kumuhana nui: ʻo ka mea mua, ka hoʻohana nui ʻana o ka tantalum chloride i ka ʻoihana a me ka noiʻi; a ʻo ka lua, pehea ka hana ʻana a me ka lawe ʻia ʻana o TaCl₅ mai nā mea maka. Hiki ke ʻike ʻia ke kūkākūkā ʻana i ka poʻe ʻaʻole loea, me ka wehewehe ʻana a me nā manaʻo o kahi e kōkua ai ke kiʻi a papaʻaina paha i ka ʻike. Ma nā wahi e hiki ai, e kuhikuhi mākou i nā kumu ʻenehana e hōʻoia i ka pololei, me ka ʻike mai nā palapala huahana loea.
Hoʻohana nui o Tantalum Chloride
ʻO ka Tantalum pentachloride kahi mea hana maʻalahiwaenaa me ka mea hooikaika. No ka mea, he ikaikaʻakika Lewis(kahi mea ʻae electron-pair), hoʻohana ʻia ʻo TaCl₅ i nā ʻano hana like ʻole a me nā kaʻina hana. ʻO kekahi mau polokalamu koʻikoʻi he:
● ʻO Catalyst i ka hoʻohuihui organik:Hana ʻo TaCl₅ ma ke ʻano he catalyst electrophilic e like me ka alumini chloride (AlCl₃). Hoʻohana ʻia ia e hāpai i nā hopena kūikawā, no ka laʻana polymerizations a i ʻoleFriedel–Craftsʻano acylations a me alkylations. Ua hoʻohana ʻia ma ke ʻano he catalyst no ka polycyclotrimerization o kekahi mau alkynes (polymer-forming reactions) a i ka hoʻomākaukau ʻana i nā pūhui chloro-aryloxide.
● ʻO ka mua o ka tantalum oxides a me ka oxychlorides:No ka mea, hoʻohāinu ʻia ʻo TaCl₅ i tantalum oxychloride (TaOCl₃) a laila i tantalum pentoxide (Ta₂O₅), hoʻohana mau ʻia ia e hana i kēlā mau mea. ʻO Ta₂O₅ kahi kīʻaha dielectric oxide i hoʻohana ʻia i nā capacitors waiwai nui a me nā uhi. I ka hoʻomaʻamaʻa ʻana, hiki ke hoʻololi ʻia ʻo TaCl₅ (ma ka hoʻohui ʻana i ka wai a i ʻole ammonia) i loko o ka tantalum oxides maʻemaʻe loa a i ʻole i loko o ka ammonium oxychloride a laila hoʻokahe ʻia i nā oxides. ʻO kēia alahele kahi kumu ʻo TaCl₅ kahi meaʻai koʻikoʻi no ka ʻoihana tantalum.
● Ka waiho ʻana o nā mea semiconductor:Ma ka ʻoihana microelectronics, hoʻohana ʻia ʻo TaCl₅ ma ke ʻano he kinoea precursor noka waiho ʻana o ka mahu (CVD)aka waiho ʻana o ka papa atomika (ALD)o nā kiʻiʻoniʻoni lahilahi me ka tantalum. No ka laʻana, hiki ke hoʻopili ʻia ka mahu TaCl₅ me ka amonia a i ʻole nā plasma nitrogen e waiho i nā kiʻiʻoniʻoni lahilahi o ka tantalum nitride (TaN), kahi mea i hoʻohana ʻia ma ke ʻano he pale diffusion a i ʻole electrode i loko o nā kaapuni hoʻohui. Hoʻohana ʻia ia e waiho i nā kiʻiʻoniʻoni tantalum pentoxide no nā capacitors. ʻO kona kūpaʻa i nā kaiapuni chlorine e kūpono ia no kēia mau kaʻina hana wela kiʻekiʻe.
● ʻO nā mea uila a me nā mea hao:ʻO ka hope loa, ua hoʻololi ʻia ka hapa nui o ka TaCl₅ i hana ʻiametala tantalumno ka hoʻohana ʻana i nā mea uila. ʻO nā capacitors Tantalum - nā capacitors liʻiliʻi i hoʻohana ʻia i nā kelepona paʻa, laptops, a me nā mea uila ʻē aʻe - hilinaʻi i Ta₂O₅ maʻemaʻe kiʻekiʻe (i loaʻa mai TaCl₅) ma ke ʻano he dielectric. ʻO TaCl₅ iho he pōhaku paepae: hiki ke hoʻemi ʻia (no ka laʻana me ka sodium a i ʻole aluminika) e hoʻohua ai i ka pauka tantalum maikaʻi, a laila hana ʻia i nā capacitors a me nā mea wela wela. I ka pōkole, ʻo TaCl₅koʻikoʻi i ka hana ʻana i ka metala tantaluma pēlā i ka ʻoihana capacitor tantalum holoʻokoʻa. (Hiki ke kōkua i ka poʻe heluhelu e nānā i kēia mau alahele ma kahi papaʻaina a i ʻole ka palapala kahe e hōʻuluʻulu ana i ka hoʻololi ʻana o TaCl₅ i mea metala, oxide, a me nitride.)
I ka hōʻuluʻulu ʻana, hoʻohana ʻia ka tantalum pentachloride ma nā wahi āpau e pono ai nā pūhui tantalum maʻemaʻe a i ʻole nā kiʻiʻoniʻoni. Hāʻawi ia i nā mea ʻeluakaʻina hana kemika organik(ma ke ano he catalyst and chlorinating agent) anā mea hana(deposition o nā kiʻiʻoniʻoni, synthesis of oxides). Wahi a ka ʻikepili mea hana, ʻo TaCl₅ "hana i mea hoʻomaka no nā pūhui hui M₆ octahedral hou i hoʻopili ʻia" a komo i ka hana ʻana i ka tantalum(V) oxychloride a me ka pentoxide. ʻO kona ʻano electrophilic (aloha electron), e like me nā catalysts maʻamau e like me AlCl₃, e hōʻike ana i kāna kuleana ma ke kemika holomua.
Pehea i hoʻomākaukau ʻia ai ʻo Tantalum Chloride
ʻO ka hana ʻana i ka tantalum pentachloride e pili ana i ka chlorinating tantalum ma kekahi ʻano. ʻElua ala nui: ka chlorination o ka tantalum metala, a me ka chlorination o nā pūhui tantalum (maʻa mau oxides). I nā hihia a pau, pono e hana ʻia ka hopena ma kahi maloʻo a me ka ʻole o ka oxygen. ʻO nā hana kumu:
● ʻO ka chlorination pololei o ka tantalum metala:Hoʻomaʻamaʻa ʻia ka metala tantalum i hoʻokaʻawale ʻia (pinepine ʻia a i ʻole ka pauka) i loko o kahi kahawai o ke kinoea chlorine. Ma kahi mahana ma kahi o 170–250 °C, hana ka chlorine me ka metala e hana i ka mahu TaCl₅:
2 Ta+5 Cl2⟶2 TaCl5.2\,Ta + 5\,Cl_2 \longrightarrow 2\,TaCl_5.
Hoʻololi koke kēia hopena exothermic i ka metala i chloride. I ka hoʻomaʻamaʻa ʻana, hoʻokomo ʻia ka tantalum i loko o kahi umu ahi a i ʻole reactor a kahe ʻia ke kinoea Cl₂ ma luna ona i ka wela hoʻomalu. Hoʻopili ʻia ka mahu TaCl₅ i loko o kahi wai a paʻa paha i kona maʻalili. (Hoʻohana kekahi ʻano hana pili i ke kinoea hydrogen chloride (HCl) ma kahi o Cl₂, akā pono kēia i ka wela kiʻekiʻe - ma kahi o 400 °C - e hoʻohuli i ka hopena.)
● ʻO ka chlorination pololei (mai nā oxides):ʻO ka pinepine, ʻaʻole hiki ke loaʻa a ʻoi aku ka pipiʻi o ka metala tantalum kiʻekiʻe. Akā, hiki i kekahi ke hoʻomaka me ka tantalum pentoxide (Ta₂O₅), ka mea i nui i loko o nā concentrates oka. Hiki ke hoʻololi ʻia ʻo Ta₂O₅ i TaCl₅ ma o ka hoʻohana ʻana i kahi mea chlorinating e like methionyl chloride (SOCl₂). ʻO ka pane:
Ta2O5+5 SOCl2→240∘C2 TaCl5+5 SO2.\text{Ta}_2\text{O}_5 + 5\,SOCl_2 \xrightarrow{240^\circ\text{C}} 2\,TaCl_5 + 5\,SO_2.
Ma kēia ʻano, ua hui pū ʻia ʻo Ta₂O₅ paʻa me ka wai SOCl₂ a hoʻomehana ʻia (ma kahi o 240 °C). Hoʻololi maikaʻi ka SOCl₂ i ka oxide i chloride, e hana ana i ke kinoea sulfur dioxide ma ke ʻano he huahana. Pono kēia ala ala ʻole i ka hana ʻana me nā pauka oxide a hiki ke hoʻohua i ka TaCl₅ maʻemaʻe loa.
Hana ʻia nā ʻano ʻelua ma lunaTaCl₅ kinoea, a laila ponohoopiliia a hoomaemaeia. I ka hoʻomaʻamaʻa ʻana, hoʻomaʻalili ʻia ke kinoea i loko o ka chlorine i hiki ai i ka TaCl₅ liquefies (kahi paila ~239 °C). Hoʻohana pinepine ʻia ka distillation e hoʻokaʻawale iā TaCl₅ mai nā mea haumia a i ʻole nā mea hoʻolapalapa haʻahaʻa. No ka laʻana, i ka synthesizing i loko o kahi lab, hiki i kekahi ke hele i ke kinoea ma o kahi pahele anu a i ʻole ke ʻano o nā condensers. Ma hope o ka condensation, hoʻomaloʻo ʻia ka huahana (hoʻomaʻamaʻa mālie ʻia ma lalo o ka momi) no ka wehe ʻana i nā ʻāpana o ka wai. Loaʻa kēia i kahi paʻa keʻokeʻo maʻemaʻe kiʻekiʻe. (ApapaʻainaʻO ka hōʻuluʻulu ʻana i kēia mau hana synthesis - ka helu ʻana i nā reactants, nā kūlana, a me nā huahana - hiki ke kōkua i ka hoʻohālikelike ʻana i nā ala i kēlā me kēia ʻaoʻao.)
● ʻO ka ʻili ʻoihana mai ka ʻeleʻele:Ma ka laulā nui, loaʻa pinepine ʻia ka tantalum mai nā minelala e like me ka tantalite a i ʻole coltan, i loaʻa nā tantalum a me ka niobium oxides. I loko o kekahi kaʻina hana ʻoihana, ua hui ʻia ka concentrate me ke kalapona (coke) a hoʻopili ʻia me ke kinoea chlorine i ka wela kiʻekiʻe. Hoʻololi kēia carbochlorination i nā oxides i nā chlorides volatile. I ka hoʻomaka ʻana, hoʻohui ʻia ka titanium, niobium, a me ka tantalum chlorides a hoʻoheheʻe i kahi wai i kapa ʻia ʻo "titanium-niobium-tantalum oxichloride." Hoʻoheheʻe ʻia kēia wai: wehe ʻia ka titanium tetrachloride (TiCl₄) mua (hoʻolapalapa ʻia 136 °C), waiho ʻia ka hapa nui o ka niobium a me ka tantalum chlorides. Hoʻopili hou ʻia ke koena hui (inā pono) e hoʻololi i nā oxychlorides i pentachlorides. ʻO ka hope loa, ua hoʻokaʻawale ʻia ka niobium chloride (NbCl₅) a me ka tantalum chloride (TaCl₅) e ka distillation fractional, no ka mea, ua paila ʻo TaCl₅ ma 239 °C a me NbCl₅ ma 248 °C. Hoʻomaʻemaʻe ʻia ka hopena hope ʻo TaCl₅. Hoʻopili pinepine ʻia kēia TaCl₅ me ka ammonia wai e hoʻoheheʻe i ka ammonium tantalum fluoride a i ʻole oxychloride, a ma ka calcination e loaʻa ai ka ultra-pure Ta₂O₅. ʻO ka mea nui, lawelawe ʻo TaCl₅ ma ke ʻano he waena i ka hoʻomaʻemaʻe ʻana i ka tantalum mai kāna ores. Apalapala kaheka hōʻike ʻana i kēia mau ʻanuʻu - mai ka ʻai maka a hiki i ka TaCl₅ a i ka oxide - he mea pono i ka poʻe heluhelu ke nānā i ke kaʻina hana ʻoihana.
I ka hōʻuluʻulu ʻana, hana ʻia ka tantalum chloride e ka halogenation o ka metala tantalum a i ʻole nā huihui. ʻO ka chlorination pololei o ka metala Ta me Cl₂ ke ala maʻalahi loa o ka lab, aʻo nā kaʻina hana ʻoihana e hoʻohana pinepine i ka chlorination kiʻekiʻe o ka tantalum oxide concentrates me ke kalapona (carbochlorination) a i ʻole me nā mea chlorinating ʻē aʻe. Hoʻopili ʻia ke kinoea TaCl₅ a hoʻoheheʻe ʻia i ka maʻemaʻe kiʻekiʻe. ʻO ka mea nui, ʻo ka ʻōlelo aʻoaʻo a kekahi mea hana e hōʻike ana ua hoʻohana ʻia ʻo TaCl₅ i ka "chlorination of organic substances" a ma ke ʻano he "chemical intermediate" i ka hana ʻana i ka metala tantalum maʻemaʻe, e hōʻike ana i kāna kuleana ma ke ʻano he reagent a me ke kī waena.


Hōʻuluʻulu manaʻo
ʻO Tantalum Chloride(TaCl₅) he mea waena kemika nui i ka ʻoihana tantalum. Hoʻohana nui ʻia ʻo ia ma ke ʻano he amea hoʻomakano ka hana ʻana i nā pūhui tantalum ʻē aʻe (oxides, nitrides, metala) a lawelawe ma ke ʻano heLewis acid catalysti loko o nā hana kemika kūikawā. Loaʻa nā noi maʻamau mai nā mea uila (tantalum capacitors, semiconductor thin films) a hiki i ka synthesis organik holomua. No ka mea, ʻo TaCl₅ ka maʻa-maʻemaʻe a me ka ʻino, pono ka mālama ʻana iā ia i nā kūlana maloʻo paʻa.
ʻO ka hana ʻana o TaCl₅ e pili ana i ka chlorinating tantalum ma kekahi ʻano. I loko o ke keʻena hana, ʻo ia hoʻi ka pane ʻana i ka metala Ta a i ʻole oxide me ka chlorine (a i ʻole nā kumu chlorine). Ma ka ʻoihana, ʻo ia ke ʻano o ka hoʻohana ʻana i ka chlorination kiʻekiʻe o ka concentrates oka, pinepine me ke kalapona, a ukali ʻia e ka distillation. Pono nā ala āpau e hoʻomaʻemaʻe pono e hoʻokaʻawale iā TaCl₅ maʻemaʻe a wehe i nā huahana.
Ka hoomaopopo ana i nahoʻohanaahana hanaʻO ka tantalum chloride he mea nui ia no ka mahalo i kāna kuleana i ka ʻenehana hou. Ma ka hoʻohui ʻana i nā kikoʻī synthesis kemika me nā noi kūpono (a me ka hāʻawi ʻana i nā mea kōkua ʻike inā he mea kōkua), hiki i ka poʻe heluhelu ke ʻike i ke ʻano o kēia pūhui i ʻike ʻole ʻia he linchpin o nā mea i hoʻokumu ʻia i ka tantalum i ka uila, kemika, a ma waho.
Ka manawa hoʻouna: Mei-30-2025