Ma waena o ka non-siliceous oxides, alumina i maikaʻi mechanical waiwai, kiʻekiʻe wela ke kū'ē a me ka corrosion kū'ē, oiai mesoporous alumina (MA) i adjustable pore nui, nui kiko'ī ili wahi, nui pore volume a me ka haʻahaʻa hana kumu kūʻai, ka mea i hoʻohana nui 'ia ma catalysis, hoʻomalu lāʻau hoʻokuʻu, adsorption a me nā kula'ē aʻe, e like me ka pohā, hydrocracking a me ka hydrodesulfurrous mea maʻamau o nā mea maʻamau. e pili pono i ka hana o ka alumina, ke ola lawelawe a me ka koho o ka catalyst. No ka laʻana, i ke kaʻina o ka hoʻomaʻemaʻe ʻana i nā kaʻa, ʻo nā mea haumia i waiho ʻia mai nā mea hoʻohui aila engine e hana i ka coke, kahi e alakaʻi ai i ka blockage o nā pores catalyst, pēlā e hōʻemi ai i ka hana o ka catalyst. Hiki ke hoʻohana i ka Surfactant e hoʻoponopono i ke ʻano o ka mea lawe alumina e hana i ka MA. Hoʻomaikaʻi i kāna hana catalytic.
He hopena koʻikoʻi ko MA, a hoʻopau ʻia nā metala ikaika ma hope o ka calcination wela kiʻekiʻe. Eia kekahi, ma hope o ka calcination kiʻekiʻe-mehana, hāʻule ka hale mesoporous, aia ka skeleton MA i ke kūlana amorphous, a ʻaʻole hiki ke hoʻokō ka acidity i kona mau koi i ke kahua o ka functionalization. Pono pinepine ka hoʻololi ʻana e hoʻomaikaʻi i ka hana catalytic, mesoporous structure stability, surface thermal stability and surface acidity of MA material. Common modification groups include metal heteroatoms (Fe, Co, Ni, Cu, Zn, Pd, Pt, Zr, etc.) a me metala oxides (TiO2, NiO, Co3O4, CuO, Cu2O, etc. iwi iwi.
ʻO ka hoʻonohonoho electron kūikawā o nā mea o ka honua i loaʻa i kāna mau pūhui i nā waiwai optical, uila a me ka magnetic, a hoʻohana ʻia i nā mea catalytic, nā mea photoelectric, nā mea adsorption a me nā mea magnetic. Hiki i nā mea mesoporous i hoʻololi ʻia ka honua ke hoʻololi i ka waiwai waika (alkali), hoʻonui i ka hakahaka o ka oxygen, a synthesize metala nanocrystalline catalyst me ka hoʻopuehu like ʻana a me ka paʻa nanometer paʻa. Ma kēia pepa, e hoʻolauna ʻia ka hoʻololi ʻana o ka honua a me ka hoʻohana ʻana o MA e hoʻomaikaʻi i ka hana catalytic, kūpaʻa wela, hiki ke mālama i ka oxygen, kikoʻī kikoʻī a me ka hoʻolālā pore.
1 MA hoomakaukau ana
1.1 hoʻomākaukau i ka lawe alumina
ʻO ke ʻano o ka hoʻomākaukau ʻana o ka mea lawe alumina e hoʻoholo ai i kāna puʻupuʻu pore, a ʻo kāna mau ʻano hoʻomākaukau maʻamau ʻo ia ke ʻano dehydration pseudo-boehmite (PB) a me ke ʻano sol-gel. Pseudoboehmite (PB) ua mua manao e Calvet, a me H + paipai peptization e loaa γ-AlOOH colloidal PB i loko o interlayer wai, i calcined a me ka dehydrated ma kiʻekiʻe wela e hana alumina. E like me nā mea maka like ʻole, ua māhele pinepine ʻia i ke ʻano o ka ua, ke ʻano carbonization a me ke ʻano hydrolysis alcoholaluminum. ʻO ka colloidal solubility o PB e pili ana i ka crystallinity, a ua hoʻonui ʻia me ka piʻi ʻana o ka crystallinity, a ua pili pū ʻia e nā kaʻina hana hana.
Hoʻomākaukau ʻia ʻo PB ma ke ʻano o ka ua. Hoʻohui ʻia ka alkali i loko o ka aluminate solution a i ʻole ka waikawa i hoʻohui ʻia i loko o ka aluminate solution a hoʻoheheʻe ʻia no ka loaʻa ʻana o ka alumina hydrated (alkali precipitation), a i ʻole hoʻohui ʻia ka waikawa i ka ua alumina e loaʻa ai ka alumina monohydrate, a laila holoi ʻia, maloʻo a calcined e loaʻa ai ka PB. He mea maʻalahi keʻano o ka ua a me ka haʻahaʻa o ke kumukūʻai, i hoʻohana pinepineʻia i ka hanaʻoihanaʻoihana, akā ua hoʻololiʻia e nā kumu he nui (pH solution, concentration, temperature, etc.). Ma ke ala carbonization, loaʻa ʻo Al(OH)3 i ka hopena o CO2 a me NaAlO2, a hiki ke loaʻa ka PB ma hope o ka ʻelemakule. Loaʻa i kēia ʻano ka maikaʻi o ka hana maʻalahi, ka maikaʻi o ka huahana kiʻekiʻe, ʻaʻohe haumia a me ke kumu kūʻai haʻahaʻa, a hiki ke hoʻomākaukau i ka alumina me ka hana catalytic kiʻekiʻe, ke kūpaʻa corrosion maikaʻi a me kahi kikoʻī kikoʻī kiʻekiʻe me ka haʻahaʻa haʻahaʻa a me ka hoʻihoʻi kiʻekiʻe. Hoʻohana pinepine ʻia ke ʻano o ka alumini alkoxide hydrolysis e hoʻomākaukau i ka PB maʻemaʻe kiʻekiʻe. Hoʻopili ʻia ka alumini alkoxide e hana i ka alumini oxide monohydrate, a laila mālama ʻia no ka loaʻa ʻana o ka PB maʻemaʻe kiʻekiʻe, nona ka crystallinity maikaʻi, ka nui o nā ʻāpana like ʻole, ka hāʻawi ʻana i ka nui o ka pore a me ka kūpaʻa kiʻekiʻe o nā ʻāpana spherical. Eia nō naʻe, paʻakikī ke kaʻina hana, a paʻakikī ke hoʻihoʻi ʻia ma muli o ka hoʻohana ʻana i kekahi mau mea hoʻoheheʻe kūlohelohe.
Eia kekahi, hoʻohana mau ʻia nā paʻakai inorganic a i ʻole nā mea hoʻohui o nā metala no ka hoʻomākaukau ʻana i nā alumina precursors ma ke ʻano sol-gel, a hoʻohui ʻia ka wai maʻemaʻe a i ʻole nā mea hoʻoheheʻe organik e hoʻomākaukau i nā hopena e hoʻohua ai i ka sol, a laila hoʻomaloʻo ʻia, maloʻo a kālua ʻia. I kēia manawa, ʻoi aku ka maikaʻi o ka hoʻomākaukau ʻana o ka alumina ma ke kumu o ka PB dehydration method, a ua lilo ke ʻano carbonization i ke ala nui no ka hana alumina ʻoihana no ka hoʻokele waiwai a me ka mālama ʻana i ke kaiapuni.
1.2 MA hoomakaukau
ʻAʻole hiki i ka alumina maʻamau ke hoʻokō i nā koi hana, no laila pono e hoʻomākaukau i ka MA kiʻekiʻe. ʻO nā ʻano hana synthesis ka mea maʻamau: nano-casting method me carbon mold as hard template; Synthesis of SDA: Evaporation-induced self-assembly process (EISA) ma ke alo o na mamana palupalu e like me SDA a me na mea cationic, anionic a i ole nonionic surfactants.
1.2.1 kaʻina EISA
Hoʻohana ʻia ke ʻano palupalu ma ke ʻano acidic, ka mea e pale ai i ke kaʻina hana paʻakikī a me ka manawa o ke ʻano membrane paʻakikī a hiki ke hoʻomaopopo i ka hoʻololi mau ʻana o ka aperture. ʻO ka hoʻomākaukau ʻana o MA e EISA ua huki nui ʻia ma muli o ka maʻalahi o ka loaʻa a me ka reproducibility. Hiki ke hoʻomākaukau ʻia nā hale mesoporous like ʻole. Hiki ke hoʻololi ʻia ka nui pore o MA ma ka hoʻololi ʻana i ka lōʻihi o ke kaulahao hydrophobic o ka surfactant a i ʻole ka hoʻoponopono ʻana i ka ratio molar o ka hydrolysis catalyst i ka alumini precursor i ka solution. etc. EISA hiki ke pani i ke kaʻina hui hui o organoaluminum precursors, e like me alumini alkoxides a me surfactant templates, typical alumini isopropoxide a me P123, no ka hoolako ana mesoporous mea.
Ma ke kaʻina hana EISA, hiki i ka hoʻohana ʻana i nā mea hoʻoheheʻe wai ʻole (e like me ka ethanol) a me nā mea hoʻonaninani kūlohelohe hiki ke hoʻolōʻihi i ka hydrolysis a me ka condensation rate o organoaluminum precursors a hoʻoulu i ka hui pū ʻana o nā mea OMA, e like me Al (OR) 3 a me alumini isopropoxide. Eia nō naʻe, i loko o nā mea hoʻoheheʻe wai ʻole, nalowale nā maʻamau surfactant i kā lākou hydrophilicity/hydrophobicity. Eia kekahi, ma muli o ka lohi o ka hydrolysis a me ka polycondensation, ʻo ka huahana waena he pūʻulu hydrophobic, kahi mea paʻakikī ke launa pū me ka surfactant template. Ke hoʻonui mālie ʻia ka neʻe ʻana o ka surfactant a me ke kiʻekiʻe o ka hydrolysis a me ka polycondensation o ka alumini i ke kaʻina o ka hoʻoheheʻe ʻana i ka solvent e hiki ai ke hui pū ʻia o ka template a me ka alumini. No laila, he nui nā ʻāpana e pili ana i nā kūlana evaporation o nā solvents a me ka hydrolysis a me ka condensation reaction o precursors, e like me ka mahana, ka haʻahaʻa pili, catalyst, solvent evaporation rate, etc. E like me ka hoike ana ma ka fig. 1, ʻO nā mea OMA me ke kūpaʻa wela kiʻekiʻe a me ka hana catalytic kiʻekiʻe i synthesized e ka solvothermal i kōkua ʻia i ka hoʻoheheʻe ʻana i hoʻokomo ʻia i ka hui pū ʻana (SA-EISA). solvothermal lapaʻau paipai i ka piha hydrolysis o alumini precursors e hana i ka liʻiliʻi puʻupuʻu alumini hui hydroxyl, i hoʻonui i ka pilina ma waena o surfactants a me alumini. Ma ke kaʻina hana EISA kuʻuna, ua hui pū ʻia ke kaʻina evaporation me ka hydrolysis o ka organoaluminum precursor, no laila he mana koʻikoʻi nā kūlana evaporation i ka hopena a me ka hoʻolālā hope o OMA. Hoʻoikaika ka hana solvothermal i ka hydrolysis piha o ka alumini precursor a hoʻopuka i nā pūʻulu alumini hydroxyl i hoʻopaʻa ʻia ʻāpana. Hoʻohālikelike ʻia me MA i hoʻomākaukau ʻia e ke ʻano EISA kuʻuna, ʻo ka OMA i hoʻomākaukau ʻia e ke ʻano SA-EISA ʻoi aku ka nui o ka nui o ka pore, ʻoi aku ka maikaʻi o ka ʻili kikoʻī a ʻoi aku ka maikaʻi o ka wela. I ka wā e hiki mai ana, hiki ke hoʻohana ʻia ke ʻano EISA no ka hoʻomākaukau ʻana i ka ultra-large aperture MA me ka helu hoʻololi kiʻekiʻe a me ke koho maikaʻi loa me ka hoʻohana ʻole ʻana i ka mea hana reaming.
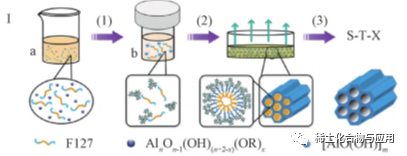
Fig
1.2.2 kaʻina hana ʻē aʻe
Pono ka hoʻomākaukau ʻana o ka MA maʻamau i ka mana pololei o nā ʻāpana synthesis e hoʻokō i kahi ʻano mesoporous maʻemaʻe, a paʻakikī hoʻi ka wehe ʻana i nā mea hoʻohālike, e hoʻopiʻi nei i ke kaʻina hana synthesis. I kēia manawa, nui nā puke i hōʻike i ka synthesis o MA me nā mamana like ʻole. I nā makahiki i hala iho nei, ua nānā nui ka noiʻi i ka synthesis o MA me ka glucose, sucrose a me ka starch e like me nā templates by aluminum isopropoxide in aqueous solution. ʻO ka hapa nui o kēia mau mea MA ka synthesized mai ka alumini nitrate, sulfate a me ka alkoxide e like me nā kumu alumini. Loaʻa iā MA CTAB ma ka hoʻololi pololei ʻana o PB ma ke kumu alumini. MA me nā waiwai hoʻolālā like ʻole, ʻo ia hoʻi ʻo Al2O3)-1, Al2O3)-2 a me al2o3A loaʻa ka paʻa wela maikaʻi. ʻAʻole hoʻololi ka hoʻohui ʻana o ka surfactant i ke ʻano aniani o ka PB, akā hoʻololi i ke ʻano hoʻopaʻa ʻana o nā ʻāpana. Eia kekahi, hoʻokumu ʻia ka hoʻokumu ʻana o Al2O3-3 e ka hoʻopili ʻana o nā nanoparticles i hoʻopaʻa ʻia e ka mea hoʻoheheʻe organik PEG a i ʻole aggregation a puni PEG. Eia naʻe, haiki loa ka puʻunaue nui o Al2O3-1. Eia kekahi, ua hoʻomākaukau ʻia nā catalysts e pili ana i ka palladium me ka MA synthetic ma ke ʻano he mea lawe.
No ka manawa mua, ua hoʻomākaukau ʻia ʻo MA me ka puʻunaue liʻiliʻi liʻiliʻi me ka hoʻohana ʻana i ka ʻeleʻele ʻeleʻele ʻeleʻele ʻeleʻele ABD. ʻO ke kaʻina hana e pili ana i ke kaʻina unuhi ma ka haʻahaʻa haʻahaʻa a me ke kaomi maʻamau. ʻAʻole hiki ke hoʻohaumia i ke kaiapuni nā ʻāpana paʻa i waiho ʻia ma ke kaʻina hana, a hiki ke hoʻopaʻa ʻia me ka haʻahaʻa haʻahaʻa a hoʻohana hou ʻia ma ke ʻano he mea hoʻopihapiha a i ʻole hoʻohui ʻia i ka noi ʻana. ʻO ka ʻāpana kikoʻī o ka MA synthesized ʻo 123 ~ 162m2 / g, He haiki ka nui o ka pore, ʻo ka radius peak he 5.3nm, a ʻo ka porosity he 0.37 cm3 / g. ʻO ka mea nano ka nui a ʻo ka nui aniani e pili ana i 11nm. ʻO ka solid-state synthesis kahi hana hou e synthesize i ka MA, hiki ke hoʻohana ʻia e hana i ka radiochemical absorbent no ka hoʻohana lapaʻau. Aluminum chloride, ammonium carbonate a me ka glucose maka mea i hui 'ia i loko o ka molar lākiō o 1: 1.5: 1.5, a MA ua synthesized e ka hou paʻa-moku'āina mechanochemical reaction.By concentrating131I i thermal lako lako, ka huina o yield of131I ma hope o ka hoʻopaʻa 'ana he 90%, a me ka radioactive loaʻa131I hoʻonā '131I. no laila e ʻike ai i ka hoʻohana ʻana i nā capsules nui dose131I[NaI] no ka mālama ʻana i ka maʻi maʻi thyroid.
I ka hōʻuluʻulu ʻana, i ka wā e hiki mai ana, hiki ke hoʻomohala ʻia nā ʻāpana molekala liʻiliʻi e kūkulu i nā hale pore i kauoha ʻia i nā pae he nui, hoʻoponopono pono i ke ʻano, morphology a me nā waiwai kemika o ka ʻili o nā mea, a hoʻohua i kahi ākea nui a kauoha i ka wormhole MA. E ʻimi i nā kumu hoʻohālike haʻahaʻa a me nā kumu alumini, e hoʻopololei i ke kaʻina hana synthesis, e wehewehe i ka mīkini synthesis a alakaʻi i ke kaʻina hana.
ʻO ke ʻano hoʻololi o 2 MA
ʻO nā ʻano o ka puʻunaue like ʻana i nā mea hana ma luna o ka mea lawe MA, ʻo ia ka impregnation, in-situ synthe-sis, precipitation, ion exchange, mechanical mixing and melting, ʻo ia nā mea ʻelua i hoʻohana pinepine ʻia.
2.1 ke ʻano hana synthesis in-situ
Hoʻohui ʻia nā hui i hoʻohana ʻia i ka hoʻololi hana i ke kaʻina hana o ka hoʻomākaukau ʻana iā MA e hoʻololi a hoʻopaʻa i ke ʻano iwi o ka mea a hoʻomaikaʻi i ka hana catalytic. Hōʻike ʻia ke kaʻina hana ma ke Kiʻi 2. Liu et al. synthesized Ni/Mo-Al2O3in situ me P123 e like me ka la'ana. Ua hoʻopuehu ʻia ʻo Ni a me Mo i nā kahawai MA i kauoha ʻia, me ka ʻole o ka luku ʻana i ke ʻano mesoporous o MA, a ua maopopo ka maikaʻi o ka hana catalytic. Ke hoʻohana nei i kahi ʻano ulu i loko o kahi gamma-al2o3substrate i hoʻohui ʻia, Hoʻohālikelike ʻia me γ-Al2O3, ʻo MnO2-Al2O3 ka nui o ka BET kikoʻī kikoʻī kikoʻī a me ka leo pore, a he bimodal mesoporous hale me ka puʻupuʻu pore haiki. MnO2-Al2O3 has fast adsorption rate and high efficiency for F-, a he ākea pH application range (pH=4~10), i kūpono no nā kūlana noiʻi ʻoihana. ʻOi aku ka maikaʻi o ka hana hou ʻana o MnO2-Al2O3 ma mua o ka γ-Al2O. I ka hōʻuluʻulu ʻana, ʻo ka MA i hoʻololi ʻia nā mea i loaʻa i ka synthesis in-situ he hoʻonohonoho hoʻonohonoho maikaʻi, ka pilina ikaika ma waena o nā pūʻulu a me nā mea lawe alumina, paʻa paʻa, hoʻoili waiwai nui, a ʻaʻole maʻalahi ke kumu i ka hoʻokahe ʻana o nā mea hana i ka hana catalytic reaction, a ʻoi aku ka maikaʻi o ka hana catalytic.
Fig. 2 Ka hoʻomākaukau ʻana o ka MA functionalized ma ka synthesis in-situ
2.2 impregnation ala
Hoʻokomo i ka MA i hoʻomākaukau ʻia i loko o ka hui i hoʻololi ʻia, a loaʻa ka mea MA hoʻololi ʻia ma hope o ka mālama ʻana, i ʻike ai i nā hopena o ka catalysis, adsorption a me nā mea like. Cai et al. hoʻomākaukau ʻo MA mai P123 ma ke ʻano sol-gel, a hoʻomoʻi iā ia i loko o ka ethanol a me ka tetraethylenepentamine solution e loaʻa ai ka amino MA hoʻololi ʻia me ka hana adsorption ikaika. Eia kekahi, ʻo Belkacemi et al. Hoʻokomo ʻia i loko o ka ZnCl2solution ma ke kaʻina hana like no ka loaʻa ʻana o ka zinc doped i hoʻololi ʻia i nā mea MA. ʻO 394m2/g a me 0.55 cm3/g ka nui o ka ʻili a me ka pore. Ke hoʻohālikelike ʻia me ke ʻano in-situ synthesis, ʻoi aku ka maikaʻi o ke ʻano o ka impregnation, ʻoi aku ka maikaʻi o ka hoʻopuehu ʻana o ka element, ka hale mesoporous paʻa a me ka hana adsorption maikaʻi, akā nāwaliwali ka ikaika o ka pilina ma waena o nā mea hana a me ka mea lawe alumina, a ua maʻalahi ka hana catalytic e nā mea o waho.
3 holomua hana
ʻO ka synthesis o rare earth MA me nā waiwai kūikawā ke ʻano hoʻomohala i ka wā e hiki mai ana. I kēia manawa, nui nā ʻano hana synthesis. Hoʻopili nā ʻāpana kaʻina i ka hana o MA. Hiki ke hoʻololi ʻia ke ʻano kikoʻī kikoʻī, ka nui o ka pore a me ke anawaena pore o MA e ke ʻano template a me ka hoʻokumu ʻana i ka alumini precursor. Hoʻopili ka mahana calcination a me ka hoʻohālikelike polimer template i ka ʻili kiko a me ka nui pore o MA. Ua ʻike ʻo Suzuki lāua ʻo Yamauchi ua hoʻonui ʻia ka mahana calcination mai 500 ℃ a 900 ℃. Hiki ke hoʻonui ʻia ka aperture a hiki ke hoʻemi ʻia ka ʻili. Eia kekahi, hoʻomaikaʻi ka hoʻololi ʻana o ka honua i ka hana, ka paʻa wela o ka ʻili, ka paʻa o ke kūkulu ʻana a me ka acidity ʻili o nā mea MA i ke kaʻina catalytic, a hoʻokō i ka hoʻomohala ʻana o ka functionalization MA.
3.1 Defluorination Adsorbent
ʻO ka fluorine i loko o ka wai inu ma Kina he mea ʻino loa ia. Eia kekahi, ʻo ka hoʻonui ʻana o ka fluorine i loko o ka ʻenehana zinc sulfate solution e alakaʻi i ka palaka o ka pā electrode, ka hoʻohaʻahaʻa ʻana o ka ʻenehana hana, ka emi ʻana o ka maikaʻi o ka zinc uila a me ka emi ʻana o ka nui o ka wai recycled i loko o ka ʻōnaehana hana waikawa a me ke kaʻina hana electrolysis o ka umu ahi e hoʻoheheʻe ʻia i ke kinoea. I kēia manawa, ʻo ke ʻano adsorption ka mea maikaʻi loa i waena o nā ʻano maʻamau o ka defluorination pulu. Akā naʻe, aia kekahi mau hemahema, e like me ka hiki ʻole o ka adsorption, haiki i loaʻa ka pae pH, pollution lua a pēlā aku. Ua hoʻohana ʻia ke kalapona, amorphous alumina, alumina hoʻāla a me nā adsorbents ʻē aʻe no ka defluorination o ka wai, akā he kiʻekiʻe ke kumukūʻai o adsorbents, a ʻo ka hiki adsorption o F-in neutral solution a i ʻole kiʻekiʻe ka hoʻopaʻa ʻana he haʻahaʻa. ka hiki o ka fluoride, a ma ka pH<6 wale no hiki ke loaʻa ka maikaʻi o ka fluoride adsorption performance.MA ua hoʻokipa ākea ka nānā ʻana i ka hoʻomalu ʻana i ka pollution kaiapuni ma muli o kona nui kikoʻī kikoʻī āpana, ʻokoʻa ka nui o ka pore hopena, ka hoʻokō ʻana i ka waika, ka thermal a me ka mechanical stability. Kundu et al. hoʻomākaukau ʻia ʻo MA me ka nui o ka fluorine adsorption hiki o 62.5 mg / g. Hoʻopili nui ʻia ka mana fluorine adsorption o MA e kona mau hiʻohiʻona, e like me kahi kikoʻī kikoʻī, nā hui hana o ka ʻili, ka nui o ka pore a me ka nui o ka pore.
Ma muli o ka ʻakika paʻakikī o La a me ke kumu paʻakikī o ka fluorine, aia kahi pilina ikaika ma waena o La a me nā ion fluorine. I nā makahiki i hala iho nei, ua ʻike kekahi mau noiʻi ʻo La ma ke ʻano he mea hoʻololi e hiki ke hoʻomaikaʻi i ka mana adsorption o ka fluoride. Eia nō naʻe, ma muli o ka haʻahaʻa haʻahaʻa o nā adsorbents honua, ʻoi aku ka nui o nā honua laha ʻole i hoʻoheheʻe ʻia i loko o ka hopena, e hopena i ka haumia wai lua a me ka pōʻino i ke olakino kanaka. Ma ka ʻaoʻao ʻē aʻe, ʻo ke kiʻekiʻe kiʻekiʻe o ka alumini i ka wai wai kekahi o nā mea make i ke olakino kanaka. No laila, pono e hoʻomākaukau i kahi ʻano adsorbent composite me ka paʻa maikaʻi a ʻaʻohe leaching a i ʻole ka liʻiliʻi o ka leaching o nā mea ʻē aʻe i ke kaʻina hana hoʻohemo fluorine. Ua hoʻomākaukau ʻia ʻo MA i hoʻololi ʻia e La a me Ce e ke ʻano impregnation (La / MA a me Ce / MA). Ua hoʻouka maikaʻi ʻia nā oxides honua ma luna o ka ili MA no ka manawa mua, ʻoi aku ka kiʻekiʻe o ka defluorination performance. The main mechanisms of fluorine removal are electrostatic adsorption and chemical adsorption, the electron attraction of surface positive charge and ligand exchange reaction combies with surface hydroxyl, the hydroxyl functional group on the adsorbent surface generates hydrogen bondification of adsorbent and the adsorbent surface. Loaʻa i ka La/MA nā kahua hoʻolaha hydroxyl adsorption, a ʻo ka mana adsorption o F aia ma ke kauoha o La/MA>Ce/MA>MA. Me ka piʻi ʻana o ka manaʻo mua, piʻi ka mana adsorption o ka fluorine. ʻOi aku ka maikaʻi o ka hopena adsorption i ka pH 5 ~ 9, a me ke kaʻina hana adsorption o ka fluorine e like me Langmuir isothermal adsorption model. Eia kekahi, hiki i nā haumia o nā ion sulfate i ka alumina ke hoʻopilikia nui i ka maikaʻi o nā laʻana. ʻOiai ua hoʻokō ʻia ka noiʻi e pili ana i ka alumina i hoʻololi ʻia i ka honua, ʻo ka hapa nui o ka noiʻi e pili ana i ke kaʻina hana o ka adsorbent, he paʻakikī ke hoʻohana ʻia i ka ʻoihana. he kumu hoʻohālike kaʻina hana no ka mālama ʻana i ka hoʻonā fluorine kiʻekiʻe e pili ana i ka honua laha ʻole MA nano adsorbent.
3.2 Mea hoʻoheheʻe
3.2.1 Hoʻoponopono maloʻo o ka methane
Hiki i ka honua laha ke hoʻololi i ka acidity (basicity) o nā mea porous, hoʻonui i ka hakahaka o ka oxygen, a synthesize catalysts me ka hoʻopuehu like, nanometer scale a me ka paʻa. Hoʻohana pinepine ʻia e kākoʻo i nā metala hanohano a me nā metala hoʻololi e hoʻopale i ka methanation o CO2. I kēia manawa, ke ulu nei nā mea mesoporous i hoʻololi ʻia i ka methane dry reforming (MDR), photocatalytic degradation o VOCs a me ka huelo kinoea hoʻomaʻemaʻe. Hoʻohālikelike ʻia me nā metala hanohano (e like me Pd, Ru, Rh, etc.) methane. Eia naʻe, ʻo ka sintering a me ka carbon deposition o Ni nanoparticles ma ka ʻili o Ni / Al2O3 alakaʻi i ka deactivation wikiwiki o ka catalyst. No laila, pono e hoʻohui i ka accelerant, hoʻololi i ka mea lawe catalyst a hoʻomaikaʻi i ke ala hoʻomākaukau e hoʻomaikaʻi i ka hana catalytic, kūpaʻa a me ka pale ʻana. Ma keʻano laulā, hiki ke hoʻohana ʻia nā oxides honua laʻa e like me nā mea hoʻolalelale a me nā mea hoʻolalelale i loko o nā catalysts heterogeneous, a hoʻomaikaʻi ʻo CeO2 i ka hoʻopuehu ʻana o Ni a hoʻololi i nā waiwai o ka metala Ni ma o ka pilina kākoʻo metala ikaika.
Hoʻohana nui ʻia ʻo MA e hoʻomaikaʻi i ka hoʻopuehu ʻana o nā metala, a hāʻawi i ke kaohi ʻana i nā metala ikaika e pale ai i kā lākou agglomeration. ʻO La2O3me ka nui o ka oxygen e mālama ai i ka hiki ke hoʻonui i ke kūpaʻa kalapona i ke kaʻina hoʻololi, a ʻo La2O3 e hoʻolaha i ka hoʻopuehu ʻana o Co ma ka alumina mesoporous, nona ka hana hoʻoponopono kiʻekiʻe a me ke kūpaʻa. Hoʻonui ka La2O3promoter i ka hana MDR o Co / MA catalyst, a ua hoʻokumu ʻia nā Co3O4and CoAl2O4phases ma ka ʻili o ka catalyst. Ma ke kaʻina hana MDR, ʻo ka pilina in-situ ma waena o La2O3 a me CO2 i hoʻokumu i ka La2O2CO3mesophase, i hoʻoulu i ka hoʻopau pono ʻana o CxHy ma ka ʻili catalyst. Hoʻoikaika ʻo La2O3 i ka hoʻemi ʻana i ka hydrogen ma o ka hāʻawi ʻana i ke kiʻekiʻe electron kiʻekiʻe a me ka hoʻonui ʻana i ka hakahaka o ka oxygen ma 10%Co/MA. ʻO ka hoʻohui ʻana o La2O3 e hōʻemi i ka ikehu hoʻāla ʻana o CH4consumption. No laila, ua piʻi ka helu hoʻololi o CH4 i 93.7% ma 1073K K. ʻO ka hoʻohui ʻana o La2O3 i hoʻomaikaʻi i ka hana catalytic, hoʻoikaika i ka hoʻemi ʻana o H2, hoʻonui i ka helu o nā wahi hana Co0, hoʻonui i ka carbon i waiho ʻia a hoʻonui i ka hakahaka o ka oxygen i 73,3%.
Ua kākoʻo ʻia ʻo Ce a me Pr ma Ni/Al2O3catalyst ma ke ʻano o ka impregnation volume like ma Li Xiaofeng. Ma hope o ka hoʻohui ʻana iā Ce a me Pr, ua hoʻonui ka koho i ka H2 a ua emi ka koho i CO. ʻO ka MDR i hoʻololi ʻia e Pr ua loaʻa ka mana catalytic maikaʻi loa, a ua piʻi ka koho i ka H2 mai 64.5% a i 75.6%, ʻoiai ua emi ka koho i CO mai 31.4% Peng Shujing et al. Ua hoʻohana ʻia ke ʻano sol-gel, ua hoʻomākaukau ʻia ʻo MA i hoʻololi ʻia me ka alumini isopropoxide, isopropanol solvent a me ka cerium nitrate hexahydrate. Ua hoʻonui iki ʻia ka ʻili kiko o ka huahana. ʻO ka hoʻohui ʻana o Ce i hōʻemi i ka hōʻuluʻulu ʻana o nā nanoparticles e like me ke koʻokoʻo ma ka ʻili MA. ʻO kekahi mau pūʻulu hydroxyl ma ka ʻili o γ- Al2O3 i uhi ʻia e nā pūhui Ce. Ua hoʻomaikaʻi ʻia ke kūpaʻa wela o MA, a ʻaʻohe hoʻololi ʻana o ke aniani kristal ma hope o ka calcination ma 1000 ℃ no nā hola 10. Wang Baowei et al. mākaukau MA mea CeO2-Al2O4by coprecipitation method. CeO2with cubic liʻiliʻi hua i like me ka hoʻopuehu i alumina. Ma hope o ke kākoʻo ʻana iā Co a me Mo ma CeO2-Al2O4, ua kāohi pono ʻia ka pilina ma waena o ka alumina a me ka mea hana Co a me Mo e CEO2.
Hoʻohui ʻia nā mea hoʻolaha honua laha (La, Ce, y a me Sm) me Co/MA catalyst no MDR, a hōʻike ʻia ke kaʻina hana ma ka fig. 3. hiki i nā mea hoʻolaha honua kakaʻikahi ke hoʻomaikaʻi i ka hoʻopuehu ʻana o Co ma luna o ka mea lawe MA a keʻakeʻa i ka hoʻohui ʻana o nā ʻāpana co. ʻoi aku ka liʻiliʻi o ka nui o ka ʻāpana, ʻoi aku ka ikaika o ka pilina Co-MA, ʻoi aku ka ikaika o ka catalytic a me ka sintering hiki i ka YCo/MA catalyst, a me nā hopena maikaʻi o kekahi mau mea hoʻolaha i ka hana MDR a me ka waiho ʻana o ke kalapona. 4 he kiʻi HRTEM ma hope o ka mālama ʻana iā MDR ma 1023K, Co2: ch4: N2 = 1 ∶ 1 ∶ 3.1 no 8 mau hola. Loaʻa nā ʻāpana Co ma ke ʻano o nā kikoʻeleʻele, ʻoiai nā mea lawe MA ma ke ʻano o ka hina, e hilinaʻi ana i ka ʻokoʻa o ka density electron. ma HRTEM kiʻi me 10%Co/MA (fig. 4b), ka agglomeration o Co metala particles ikeia ma ma carriersThe hoʻohui o rare earth promoter hoemi Co particles i 11.0nm~12.5nm. Loaʻa i ka YCo/MA ka pilina Co-MA ikaika, a ʻoi aku ka maikaʻi o kāna hana sintering ma mua o nā catalysts. Eia kekahi, e like me ka hoikeia ma na fiku. 4b a hiki i 4f, hollow carbon nanowires (CNF) i hana ʻia ma nā catalysts, e hoʻopaʻa mau ana me ke kahe kinoea a pale i ka catalyst mai ka deactivation.

Fig. 3 Ka hopena o ka hoʻohui ʻia ʻana o ka honua ma luna o nā waiwai kino a me nā waiwai kemika a me ka hana catalytic MDR o Co/MA catalyst.
3.2.2 Deoxidation catalyst
ʻO Fe2O3 / Meso-CeAl, he mea hoʻoheheʻe deoxidation ma muli o Ce-doped Fe, ua hoʻomākaukau ʻia e ka oxidative dehydrogenation o 1- butene me CO2as soft oxidant, a ua hoʻohana ʻia i ka synthesis o 1,3- butadiene (BD). Ua hoʻopuehu nui ʻia ʻo Ce i loko o ka alumina matrix, a ua hoʻopuehu nui ʻia ʻo Fe2O3/mesoFe2O3/Meso-CeAl-100 catalyst ʻaʻole wale nō i hoʻopuehu nui ʻia nā ʻano hao a me nā waiwai structural maikaʻi, akā loaʻa nō hoʻi ka mana mālama oxygen maikaʻi, no laila ua loaʻa iā ia ka adsorption maikaʻi a me ka hiki ke hoʻāla ʻia o CO2. E like me ka mea i hōʻike ʻia ma ka Figure 5, hōʻike nā kiʻi TEM i ka Fe2O3/Meso-CeAl-100 maʻamau. Ua hoʻomohala ka mea hoʻoheheʻe metala maikaʻi i ke kūlana haʻahaʻa haʻahaʻa loa o nā kaʻa kaʻa i ka hoʻolālā pore, maikaʻi hydrothermal kūpaʻa a me ka nui o ka mālama ʻana i ka oxygen.
3.2.3 Hoʻoikaika no nā kaʻa
Kākoʻo ʻo Pd-Rh i nā paʻina honua laha ʻole ma ka quaternary aluminika AlCeZrTiOx a me AlLaZrTiOx no ka loaʻa ʻana o nā mea hoʻoheheʻe catalyst kaʻa. Hiki ke hoʻohana maikaʻi ʻia ka mesoporous aluminum-based rare earth complex Pd-Rh/ALC ma ke ʻano he CNG kaʻa hoʻomaʻemaʻe hoʻomaʻemaʻe catalyst me ka lōʻihi maikaʻi, a me ka hoʻololi ʻana o CH4, ka mea nui o ka CNG exhaust gas, he kiʻekiʻe e like me 97.8%. Hoʻohana i ka hydrotherMAl hoʻokahi-ʻanuʻu kaʻina hana e hoomakaukau i ka honua maʻamau ma composite mea e ike iho-hui, Kauoha mesoporous precursors me metastable moku'āina a me ka kiʻekiʻe aggregation ua synthesized, a me ka synthesis o RE-Al conformed i ke kumu hoʻohālike o "hui hui ulu", pela e ike i ka hoʻomaʻemaʻe o kaʻa pauku-hoʻohuehu post-amounted.
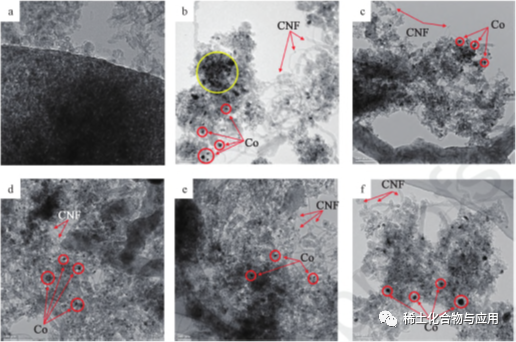
Fig. 4 HRTEM kiʻi o ma (a), Co/ MA(b), LaCo/MA(c), CeCo/MA(d), YCo/MA(e) a me SmCo/MA(f)
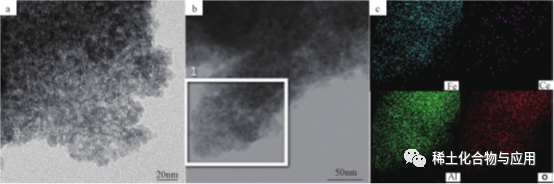
Fig. 5 kiʻi TEM (A) a me ka diagram element EDS (b,c) o Fe2O3/Meso-CeAl-100
3.3 hana kukui
Hiki ke hauʻoli nā electron o nā mea honua laha ʻole i ka hoʻololi ʻana ma waena o nā pae ikehu like ʻole a hoʻokuʻu i ka mālamalama. Hoʻohana pinepine ʻia nā ion o ka honua ma ke ʻano he mea hana e hoʻomākaukau ai i nā mea luminescent. Hiki ke hoʻouka ʻia nā ion honua laha ʻole ma ka ʻili o ka aluminika phosphate hollow microspheres ma ke ʻano corecipitation a me ke ʻano hoʻololi ion, a hiki ke hoʻomākaukau ʻia nā mea luminescent AlPO4∶RE(La,Ce,Pr,Nd). ʻO ka lōʻihi o ka nalu luminescent ma kahi kokoke i ultraviolet. Hana ʻia ʻo MA i nā kiʻiʻoniʻoni lahilahi ma muli o kona inertia, haʻahaʻa dielectric mau a me ka haʻahaʻa conductivity, kahi e pili ai i nā mea uila a me ka optical, nā kiʻi ʻoniʻoni ʻoniʻoni, nā pale, nā mea ʻike, a me nā mea ʻē aʻe. Hoʻopaʻa ʻia kēia mau mea hana i nā kiʻiʻoniʻoni me ka lōʻihi o ke ala optical definite, no laila, pono e hoʻomalu i ka refractive index a me ka mānoanoa. I kēia manawa, hoʻohana pinepine ʻia ka titanium dioxide a me ka zirconium oxide me ka index refractive kiʻekiʻe a me ka silicon dioxide me ka index refractive haʻahaʻa e hoʻolālā a kūkulu i ia mau mea. Hoʻonui ʻia ka loaʻa ʻana o nā mea me nā waiwai kemika o ka ʻili, kahi e hiki ai ke hoʻolālā i nā mea ʻike photon kiʻekiʻe. ʻO ka hoʻokomoʻana i nā kiʻiʻoniʻoni MA a me ka oxyhydroxide i ka hoʻolālāʻana i nā meaʻikeʻike e hōʻike ana i ka mana nui no ka mea ua like ka index refractive me ka silicon dioxide. Akā, heʻokoʻa nā mea kemika.
3.4 paʻa wela
Me ka piʻi ʻana o ka mahana, hoʻololi koʻikoʻi ka sintering i ka hopena hoʻohana o ka catalyst MA, a ke emi nei ka ʻili kikoʻī a me ka γ-Al2O3in crystalline phase transforms i δ a me θ i ka χ. Loaʻa i nā mea ʻāina laha ʻole ke kūpaʻa kemika maikaʻi a me ke kūpaʻa wela, ka hiki ke hoʻololi kiʻekiʻe, a me ka maʻalahi o ka loaʻa ʻana o nā mea maka. ʻO ka hoʻohui ʻana i nā mea ʻenehana honua hiki ke hoʻomaikaʻi i ke kūpaʻa wela, ke kūpaʻa ʻana o ka wela wela a me nā waiwai mechanical o ka mea lawe, a hoʻoponopono i ka acidity ili o ka mea lawe. ʻO La a me Ce nā mea hoʻololi maʻamau a aʻo ʻia. Ua ʻike ʻo Lu Weiguang a me nā mea ʻē aʻe ʻo ka hoʻohui ʻana o nā mea ʻenekele honua i pale maikaʻi i ka hoʻopuehu nui ʻana o nā ʻāpana alumina, La a me Ce i pale i nā pūʻulu hydroxyl ma ka ʻili o ka alumina, kāohi i ka sintering a me ka hoʻololi ʻana o ka pae, a hoʻemi i ka pōʻino o ka wela kiʻekiʻe i ka hale mesoporous. ʻO ka alumina i hoʻomākaukau ʻia he kiʻekiʻe ka nui o ka ʻili a me ka pore volume. Akā naʻe, ʻoi aku ka nui a i ʻole ka liʻiliʻi liʻiliʻi o ka honua mea e hōʻemi ai i ke kūpaʻa wela o ka alumina. Li Yanqiu et al. ua hoʻohui ʻia ʻo 5% La2O3to γ-Al2O3, ka mea i hoʻomaikaʻi i ke kūpaʻa wela a hoʻonui i ka nui o ka pore a me kahi kikoʻī kikoʻī o ka mea lawe alumina. E like me ka mea i ʻike ʻia mai ke Kiʻi 6, ua hoʻohui ʻia ʻo La2O3 i ka γ-Al2O3, E hoʻomaikaʻi i ke kūpaʻa wela o ka mea hoʻokomo i ka honua laha.
Ma ke kaʻina hana o ka doping nano-fibrous particles me La i MA, ka BET ili wahi a me ka pore leo o MA-La ua oi aku mamua o ka poe o MA ke hoʻonui ka wela lapaʻau wela, a me ka doping me La i maopopo retarding hopena ma ka sintering ma kiʻekiʻe wela. e like me ka hoikeia ma ka fig. 7, me ka piʻi ʻana o ka mahana, La inhibits i ka hopena o ka ulu ʻana o ka palaoa a me ka hoʻololi ʻana o ka pae, ʻoiai nā fig. Hōʻike ka 7a a me 7c i ka hōʻiliʻili ʻana o nā ʻāpana nano-fibrous. ma fig. 7b, ʻo ke anawaena o nā ʻāpana nui i hana ʻia e ka calcination ma 1200 ℃ ma kahi o 100nm. Hōʻailona ia i ka sintering koʻikoʻi o MA. Eia kekahi, ke hoʻohālikelike ʻia me MA-1200, ʻaʻole i hōʻuluʻulu ʻo MA-La-1200 ma hope o ka mālama ʻana i ka wela. Me ka hoʻohui ʻana o La, ʻoi aku ka maikaʻi o ka sintering i nā ʻāpana nano-fiber. ʻoiai ma ka mahana calcination kiʻekiʻe, ua hoʻopuehu nui ʻia ka doped La ma ka ʻili MA. Hiki ke hoʻohana ʻia ʻo La modified MA ma ke ʻano he mea lawe o Pd catalyst i ka hopena C3H8oxidation.
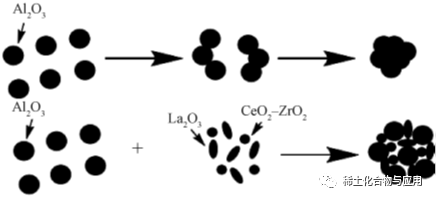
Fig. 6 Ke kumu hoʻohālike o ka alumina sintering me ka ʻole o nā mea honua laha ʻole

7 Nā kiʻi TEM o MA-400 (a), MA-1200(b), MA-La-400(c) a me MA-La-1200(d)
4 Ka hopena
Hoʻokomo ʻia ka holomua o ka hoʻomākaukau ʻana a me ka hoʻohana ʻana i nā mea MA i hoʻololi ʻia i ka honua. Hoʻohana nui ʻia ka MA i hoʻololi ʻia i ka honua. ʻOiai ua hana ʻia ka nui o ka noiʻi ma ka noi catalytic, thermal stability a me adsorption, nui nā mea waiwai i kiʻekiʻe ke kumu kūʻai, haʻahaʻa doping nui, maikaʻi ʻole a paʻakikī i ka hana ʻana. Pono e hana ʻia kēia mau hana i ka wā e hiki mai ana: hoʻomaikaʻi i ka haku mele ʻana a me ke ʻano o ka honua i hoʻololi ʻia MA, koho i ke kaʻina hana kūpono, E hālāwai me ka hoʻomohala hana; E hoʻokumu i kahi hiʻohiʻona kaʻina hana e pili ana i ka hana hana e hōʻemi i nā kumukūʻai a hoʻomaopopo i ka hana ʻoihana; I mea e hoʻonui ai i ka maikaʻi o nā kumuwaiwai honua laha ʻole o Kina, pono mākou e ʻimi i ke ʻano o ka hoʻololi ʻana o ka honua rare MA, hoʻomaikaʻi i ke kumumanaʻo a me ke kaʻina hana o ka hoʻomākaukau ʻana i ka honua rare MA hoʻololi ʻia.
Ka Papahana Waiwai: Shaanxi Science and Technology Overall Innovation Project (2011KTDZ01-04-01); ʻO Shaanxi Province 2019 Special Scientific Research Project (19JK0490); 2020 papahana noiʻi ʻepekema kūikawā o Huaqing College, Xi 'an University of Architecture and Technology (20KY02)
Puna: Rare Earth
Ka manawa hoʻouna: Jul-04-2022